PRA number
Published date
Reasons the product is recalled
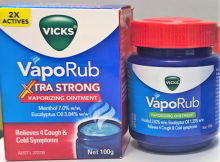
Some 100g jars of Vicks VapoRub Xtra Strong in batch number 222605 have been incorrectly labelled as Vicks VapoRub.
The hazards to consumers
Use of Vicks Vaporub Xtra Strong may cause allergic reactions (rash, blister, hives, peeling, itching or swelling) in some consumers.
Steam inhalation by 6-10 year old children may result in an increased risk of adverse events (ocular irritation, headache, dizziness, nausea, and tachycardia).
What consumers should do
Check the packaging carton or the jar label for the affected batch number. Alternatively, the affected product will have a VapoRub Xtra Strong red lid and the standard Vicks VapoRub ointment 100g label with a green element (as pictured).
If you have a jar of Vicks VapoRub Xtra Strong 100g from batch 222605, do not use it.
Return it to the place of purchase for a refund or call our customer service line to arrange the return of affected product and refund.
For more details see https://apps.tga.gov.au/Prod/sara/arn-detail.aspx?k=RC-2022-RN-01398-1
For further information contact Customer Service by phone on 1800 028 280.
Traders who sold this product
Chemist Warehouse and My Chemist stores in NSW, Vic and TAS
Where the product was sold
Dates available for sale
Recall advertisements and supporting documentation
Responsible regulator
Therapeutic Goods Administration is the responsible regulator for this recall.